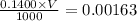Chemistry, 14.04.2020 21:08 Qwerty2771
A chemistry student weighs out 0.154 g of chloroacetic acid (HCH2CICO2) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1400 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equivalence point.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

Chemistry, 23.06.2019 10:30
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1

Chemistry, 23.06.2019 13:40
Which of the following volumes is the smallest? a) one microliter b)one deciliter d)one liter c)one milliliter
Answers: 2
You know the right answer?
A chemistry student weighs out 0.154 g of chloroacetic acid (HCH2CICO2) into a 250. mL volumetric fl...
Questions


Mathematics, 25.08.2019 17:30




Health, 25.08.2019 17:30

Mathematics, 25.08.2019 17:30

Mathematics, 25.08.2019 17:30

Social Studies, 25.08.2019 17:30

Physics, 25.08.2019 17:30


Mathematics, 25.08.2019 17:30

Mathematics, 25.08.2019 17:30


English, 25.08.2019 17:30


Mathematics, 25.08.2019 17:30

Mathematics, 25.08.2019 17:30

Mathematics, 25.08.2019 17:30

Social Studies, 25.08.2019 17:30


 moles of chloroacetic acid
moles of chloroacetic acid moles
moles