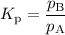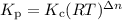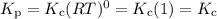Chemistry, 14.04.2020 21:01 damari9288
Select the statements below that are TRUE. Kc has units of molarity and Kp has units of pressure Kp uses the same equation as Kc, but with pressure instead of concentration If there is a liquid in a reaction, you use the vapor press for that entry in the Kp equation. Kc and Kp are the same thing as the k (rate constant) that you learned about before. Kc is equal to Kp when the number of moles of gas (n) doesn't change. Solids and liquid are not included in the Kc and Kp equations because they have an activity equal to 1.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1
You know the right answer?
Select the statements below that are TRUE. Kc has units of molarity and Kp has units of pressure Kp...
Questions






History, 06.09.2020 05:01

Mathematics, 06.09.2020 05:01

Physics, 06.09.2020 05:01


Mathematics, 06.09.2020 05:01

Chemistry, 06.09.2020 05:01

Mathematics, 06.09.2020 05:01


Computers and Technology, 06.09.2020 05:01

Mathematics, 06.09.2020 05:01




Mathematics, 06.09.2020 05:01

Computers and Technology, 06.09.2020 05:01

![K_{\text{c}} = \dfrac{\text{[B]}}{\text{[A]}}](/tpl/images/0599/2268/ba231.png)