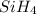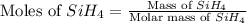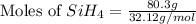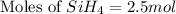Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
The burning of 80.3 g of SiH4 at constant pressure gives off 3790 kJ of heat. Calculate △H for this...
Questions


Mathematics, 10.02.2021 21:10


Mathematics, 10.02.2021 21:10


Spanish, 10.02.2021 21:10

Mathematics, 10.02.2021 21:10

Mathematics, 10.02.2021 21:10


Mathematics, 10.02.2021 21:10


Mathematics, 10.02.2021 21:10


Mathematics, 10.02.2021 21:10


Mathematics, 10.02.2021 21:10

Mathematics, 10.02.2021 21:10

Mathematics, 10.02.2021 21:10