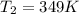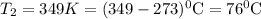Mount Everest rises to a height of 8,850 m above sea level. At a base camp on the mountain the atmospheric pressure is measured to be 314.0 mm Hg. At what temperature (in °C) will water boil at base camp ? The vapor pressure of water at 373 K is 760.0 mm Hg. (ΔH°vap for H2O = 40.7 kJ/mol and R = 8.314 J/mol K).
a. 344°C.
b. 70.8°C .
c. 2.91E-3°C .
d. 79.8°C.
e. 57.8°C.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
Mount Everest rises to a height of 8,850 m above sea level. At a base camp on the mountain the atmos...
Questions


Mathematics, 22.02.2021 17:40

Social Studies, 22.02.2021 17:40



Mathematics, 22.02.2021 17:40


History, 22.02.2021 17:40

Mathematics, 22.02.2021 17:40

Social Studies, 22.02.2021 17:40

Social Studies, 22.02.2021 17:40



Mathematics, 22.02.2021 17:40

English, 22.02.2021 17:40

Mathematics, 22.02.2021 17:40

Social Studies, 22.02.2021 17:40



Mathematics, 22.02.2021 17:40

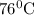 .
.![ln(\frac{P_{2}}{P_{1}})=\frac{-\Delta H_{vap}^{0}}{R}[\frac{1}{T_{2}}-\frac{1}{T_{1}}]](/tpl/images/0599/0769/06906.png)
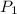 and
and 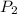 are vapor pressures of liquid at
are vapor pressures of liquid at 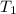 (in kelvin) and
(in kelvin) and 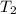 (in kelvin) temperatures respectively.
(in kelvin) temperatures respectively.![ln(\frac{314.0}{760.0})=\frac{-40.7\times 10^{3}\frac{J}{mol}}{8.314\frac{J}{mol.K}}\times [\frac{1}{T_{2}}-\frac{1}{373K}]](/tpl/images/0599/0769/811c1.png)