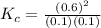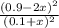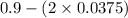Chemistry, 14.04.2020 19:52 KariSupreme
At equilibrium, the concentrations in this system were found to be [ N 2 ] = [ O 2 ] = 0.100 M [N2]=[O2]=0.100 M and [ NO ] = 0.600 M . [NO]=0.600 M. N 2 ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO ( g ) N2(g)+O2(g)↽−−⇀2NO(g) If more NO NO is added, bringing its concentration to 0.900 M, 0.900 M, what will the final concentration of NO NO be after equilibrium is re‑established?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 12:50
Acertain reaction has a activation energy of 54.0 kj/mol. as the temperature is increased from 22c to a higher temperature, the rate constant increases by a factor of 7.00. calculate the higher temperature. c (report only numerical answer)
Answers: 3
You know the right answer?
At equilibrium, the concentrations in this system were found to be [ N 2 ] = [ O 2 ] = 0.100 M [N2]=...
Questions

Biology, 02.01.2021 14:00

Social Studies, 02.01.2021 14:00

Social Studies, 02.01.2021 14:00

Mathematics, 02.01.2021 14:00

English, 02.01.2021 14:00

English, 02.01.2021 14:00

English, 02.01.2021 14:00


Computers and Technology, 02.01.2021 14:00


Mathematics, 02.01.2021 14:00

Mathematics, 02.01.2021 14:00

Mathematics, 02.01.2021 14:00

Mathematics, 02.01.2021 14:00

Physics, 02.01.2021 14:00

Mathematics, 02.01.2021 14:00

Mathematics, 02.01.2021 14:00

Chemistry, 02.01.2021 14:00

English, 02.01.2021 14:00

Business, 02.01.2021 14:00

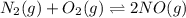
![K_{c} = \frac{[NO]^{2}}{[N_{2}][O_{2}]}](/tpl/images/0599/0103/a7a07.png)
![[N_{2}] = [O_{2}]](/tpl/images/0599/0103/c9b74.png) = 0.1 M and [NO] = 0.6 M
= 0.1 M and [NO] = 0.6 M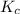 is as follows.
is as follows.