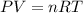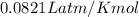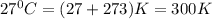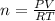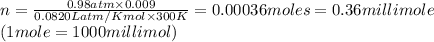If the barometric pressure that day was 75.1 cm Hg, the temperature was 27.0°C and the volume in the pipette decreases from 51.0 mL to 42.0 mL, how many millimoles of gas (total) were removed from the initial air sample? (Enter as a decimal value, not in scientific notation and enter the value only. Note that a millimole is 10-3 mole.)

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1

Chemistry, 23.06.2019 10:30
Me soon im confused much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 1
You know the right answer?
If the barometric pressure that day was 75.1 cm Hg, the temperature was 27.0°C and the volume in the...
Questions

Arts, 02.09.2020 19:01






History, 02.09.2020 19:01


English, 02.09.2020 19:01

Physics, 02.09.2020 19:01

Biology, 02.09.2020 19:01


Mathematics, 02.09.2020 19:01



Computers and Technology, 02.09.2020 19:01




English, 02.09.2020 19:01