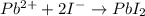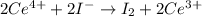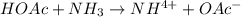Chemistry, 14.04.2020 18:48 luzinaustin
The following reactions: Pb2+ + 2I– → PbI2 2Ce4+ + 2I– → I2 + 2Ce3+ HOAc + NH3 → NH4+ + OAc– are examples of
a. acid-base reactions
b. unbalanced reactions
c. precipitation, acid-base, and redox reactions, respectively
d. redox, acid-base, and precipitation reactions, respectively
e. precipitation, redox, and acid-base reactions, respectively

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
The following reactions: Pb2+ + 2I– → PbI2 2Ce4+ + 2I– → I2 + 2Ce3+ HOAc + NH3 → NH4+ + OAc– are exa...
Questions


English, 05.10.2019 05:30

Chemistry, 05.10.2019 05:30

Mathematics, 05.10.2019 05:30




Business, 05.10.2019 05:30



Mathematics, 05.10.2019 05:30

Social Studies, 05.10.2019 05:30

Mathematics, 05.10.2019 05:30

English, 05.10.2019 05:30



English, 05.10.2019 05:30


Computers and Technology, 05.10.2019 05:30

Health, 05.10.2019 05:30