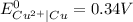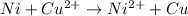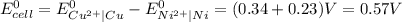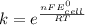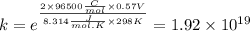Use standard reduction potentials to calculate the equilibrium constant for the reaction:
Cu²...

Chemistry, 14.04.2020 18:32 lovemichelle638
Use standard reduction potentials to calculate the equilibrium constant for the reaction:
Cu²⁺ (aq) + Ni(s) → Cu(s) + Ni²⁺ (aq)
Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
Questions

English, 10.05.2021 14:10

Mathematics, 10.05.2021 14:10

English, 10.05.2021 14:10

Mathematics, 10.05.2021 14:10



History, 10.05.2021 14:10


Mathematics, 10.05.2021 14:10

Mathematics, 10.05.2021 14:10

History, 10.05.2021 14:10

Mathematics, 10.05.2021 14:10



Mathematics, 10.05.2021 14:10

Mathematics, 10.05.2021 14:10

Mathematics, 10.05.2021 14:10

Mathematics, 10.05.2021 14:10

Mathematics, 10.05.2021 14:10

Chemistry, 10.05.2021 14:10


 ;
; 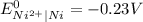
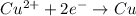 ;
;