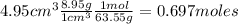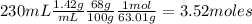Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and photochemical smog. What volume (in L) of nitrogen dioxide is formed at 735 torr and 28.2°C by reacting 4.95 cm3 of copper (d = 8.95 g/cm3 ) with 230.0 mL of nitric acid (d = 1.42 g/cm3 , 68.0% HNO3 by mass)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2


Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and ph...
Questions

Mathematics, 09.12.2021 08:30

Mathematics, 09.12.2021 08:30


Mathematics, 09.12.2021 08:30

History, 09.12.2021 08:30

Spanish, 09.12.2021 08:30


Spanish, 09.12.2021 08:30


Mathematics, 09.12.2021 08:30

Mathematics, 09.12.2021 08:30



Mathematics, 09.12.2021 08:30


Mathematics, 09.12.2021 08:30

Mathematics, 09.12.2021 08:30

History, 09.12.2021 08:30

Mathematics, 09.12.2021 08:30