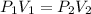Chemistry, 14.04.2020 16:33 lindasuebairdoyjpf7
A sample of an ideal gas at 1.00 atm and a volume of 1.87 L was placed in a weighted balloon and dropped into the ocean. As the sample descended, the water pressure compressed the balloon and reduced its volume. When the pressure had increased to 80.0 atm , what was the volume of the sample

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

You know the right answer?
A sample of an ideal gas at 1.00 atm and a volume of 1.87 L was placed in a weighted balloon and dro...
Questions

Chemistry, 10.05.2021 01:30

Mathematics, 10.05.2021 01:30

Health, 10.05.2021 01:30

Chemistry, 10.05.2021 01:30

Mathematics, 10.05.2021 01:30

Mathematics, 10.05.2021 01:30



Mathematics, 10.05.2021 01:30

Mathematics, 10.05.2021 01:30


Physics, 10.05.2021 01:30

Computers and Technology, 10.05.2021 01:30


Physics, 10.05.2021 01:30



Physics, 10.05.2021 01:30


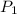 and
and 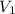 are the initial gas parameter before dropping into the ocean
are the initial gas parameter before dropping into the ocean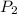 and
and 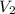 are the final gas parameter after dropping into the ocean
are the final gas parameter after dropping into the ocean