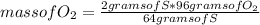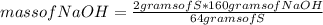Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Which substance is the limiting reactant when 2.0 g of sulfur reacts with 3.0 g of oxygen and 4.0 g...
Questions



Mathematics, 11.03.2020 06:23




Mathematics, 11.03.2020 06:24


Social Studies, 11.03.2020 06:24





History, 11.03.2020 06:24

English, 11.03.2020 06:24


Mathematics, 11.03.2020 06:25