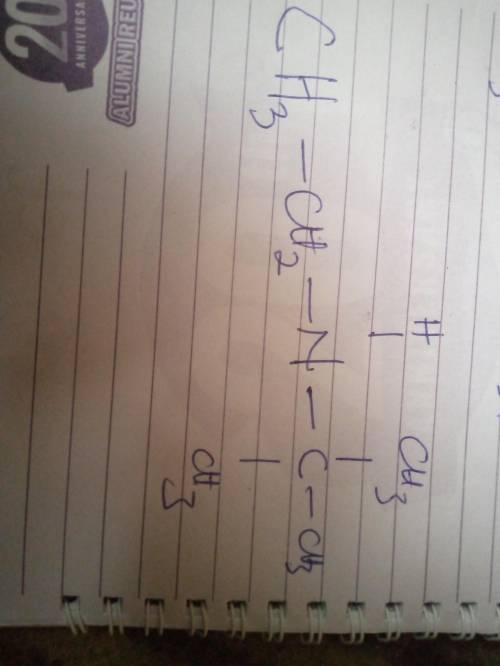
A compound with molecular formula C6H15N exhibits a singlet at d 0.9 ppm (1H), a triplet at 1.10 ppm (3H), a singlet at 1.15 ppm (9H), and a quartet at 2.6 ppm (2H) in its 1HNMR spectrum. Its IR spectrum shows one medium absorption band near 3400 cm-1. What is the structure of this compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
A compound with molecular formula C6H15N exhibits a singlet at d 0.9 ppm (1H), a triplet at 1.10 ppm...
Questions


Mathematics, 25.09.2020 01:01

Computers and Technology, 25.09.2020 01:01


Mathematics, 25.09.2020 01:01


Computers and Technology, 25.09.2020 01:01



Geography, 25.09.2020 01:01

Biology, 25.09.2020 01:01




English, 25.09.2020 01:01

Mathematics, 25.09.2020 01:01

English, 25.09.2020 01:01