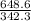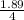Chemistry, 13.04.2020 22:49 20warriorsoul14
If 684.6g of sucrose (table sugar) are dissolved in 4 liters of water, what would be the molarity of the solution?
The chemical formula for sucrose is C12H22O11 , and the molar mass is 342.3 g/mol. mol/L

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 08:00
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1
You know the right answer?
If 684.6g of sucrose (table sugar) are dissolved in 4 liters of water, what would be the molarity of...
Questions

Social Studies, 28.08.2019 15:50

English, 28.08.2019 15:50


English, 28.08.2019 15:50


Advanced Placement (AP), 28.08.2019 15:50

English, 28.08.2019 15:50

Biology, 28.08.2019 15:50

Business, 28.08.2019 15:50





English, 28.08.2019 15:50

History, 28.08.2019 15:50





Social Studies, 28.08.2019 15:50

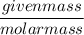
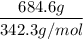
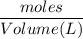

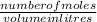 equation 1
equation 1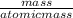 equation 2
equation 2