6. which of the following statements is true?
A. in an exothermic reaction, the energy of the...

6. which of the following statements is true?
A. in an exothermic reaction, the energy of the products is the same as the energy of the reactants.
B. in an endothermic reaction, the energy of the products is the same as the energy of the reactants.
C. in an exothermic reaction, the energy of the products is less than the energy of the reactance.
D. in an endothermic reaction, the energy of the products is less than the energy of the reactants.
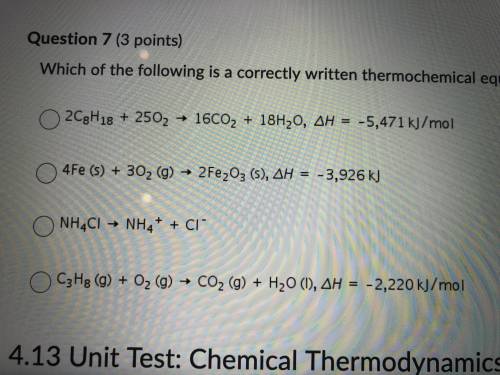
7. which of the following is correctly written thermochemical equation?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

You know the right answer?
Questions


History, 13.01.2021 18:50








History, 13.01.2021 18:50


Mathematics, 13.01.2021 18:50


Mathematics, 13.01.2021 18:50

Mathematics, 13.01.2021 18:50


Mathematics, 13.01.2021 18:50

Mathematics, 13.01.2021 18:50




