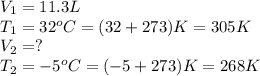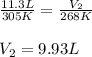Chemistry, 13.04.2020 21:22 gfdsgfd5654
A sample of hydrogen gas at a pressure of 977 mm Hg and a temperature of 32 °C, occupies a volume of 11.3 liters. If the gas is cooled at constant pressure to a temperature of -5 °C, the volume of the gas sample will be L.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
A sample of hydrogen gas at a pressure of 977 mm Hg and a temperature of 32 °C, occupies a volume of...
Questions

Mathematics, 17.08.2021 01:10

Mathematics, 17.08.2021 01:10

Chemistry, 17.08.2021 01:10





Mathematics, 17.08.2021 01:10

Mathematics, 17.08.2021 01:10



Arts, 17.08.2021 01:10

Computers and Technology, 17.08.2021 01:10

Mathematics, 17.08.2021 01:10

Mathematics, 17.08.2021 01:10



Mathematics, 17.08.2021 01:10

English, 17.08.2021 01:10

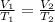
 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.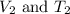 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.