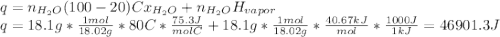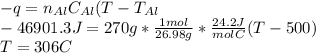Chemistry, 11.04.2020 04:49 goreeefk4939
18.1 g of water (initially at 20 oC) is poured onto 270 g of hot, aluminum metal (initially at 500 oC). Once all of the water has vaporized (and no heat is lost to the surrounding air) what will be the final temperature of the aluminum?
a. MPAl = 660 oC
b. Csolid Al = 24.2 J/mol oC
c. Cliquid Al = 29.3 J/mol oC
d. Cliquid water = 75.3 J/mol oC
e. Cwater vapor = 33.1 J/mol oC
f.ΔHfus water = 6.02 kJ/mol
g.ΔHvap water = 40.67 kJ/mol

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
18.1 g of water (initially at 20 oC) is poured onto 270 g of hot, aluminum metal (initially at 500 o...
Questions

Arts, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20


Physics, 11.05.2021 20:20

Biology, 11.05.2021 20:20



Mathematics, 11.05.2021 20:20

Chemistry, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

English, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

Mathematics, 11.05.2021 20:20

History, 11.05.2021 20:20


Mathematics, 11.05.2021 20:20