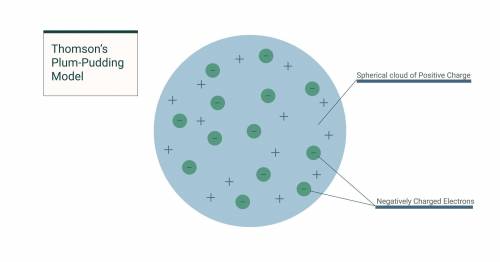
Which of the following answer choices best describes J. J. Thomson's plum-pudding model?An atom is surrounded by a firm outer shell and contains positively charged particles in its core. An atom is surrounded by a firm outer shell and contains negatively charged particles in its core. An atom consists of positively charged matter that contains negatively charged particles. An atom consists of negatively charged matter that contains positively charged particles.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Which of the following answer choices best describes J. J. Thomson's plum-pudding model?An atom is s...
Questions



Mathematics, 23.05.2021 14:00

English, 23.05.2021 14:00


Physics, 23.05.2021 14:00



Mathematics, 23.05.2021 14:00

English, 23.05.2021 14:00

English, 23.05.2021 14:00

Biology, 23.05.2021 14:00


Mathematics, 23.05.2021 14:00


Computers and Technology, 23.05.2021 14:00

Mathematics, 23.05.2021 14:00



Mathematics, 23.05.2021 14:00