
Chemistry, 10.04.2020 05:16 kragland4752
Calculate the volume of 0.500 M HCOOH and 0.500 M HCOONa required to prepare 0.100 L of pH = 4.25 buffer with a buffer
strength of 0.100 M. The pk, of HCOOH is 3.75.
нсоон:
HCOONa:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Calculate the volume of 0.500 M HCOOH and 0.500 M HCOONa required to prepare 0.100 L of pH = 4.25 bu...
Questions


English, 18.07.2020 22:01




Social Studies, 18.07.2020 22:01












Arts, 18.07.2020 22:01


Mathematics, 18.07.2020 22:01

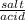
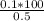 = 20 is the second equation.
= 20 is the second equation.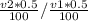 = 5.62
= 5.62

