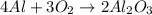Chemistry, 10.04.2020 04:56 katiedurden02
A 0.191g piece of solid aluminum reacts with gaseous oxygen from the atmosphere to form solid aluminum oxide. In the laboratory, a student weighs the mass of the aluminum oxide collected from this reaction as 0.199 g. The 0.191g solid aluminum is the .A) percent yield,
B) excess reagent,
C) limiting reagent,
D) actual yield,
E) theoretical yield

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
A 0.191g piece of solid aluminum reacts with gaseous oxygen from the atmosphere to form solid alumin...
Questions


English, 24.07.2019 13:30



Chemistry, 24.07.2019 13:30

Business, 24.07.2019 13:30

Biology, 24.07.2019 13:30

Mathematics, 24.07.2019 13:30


Biology, 24.07.2019 13:30

Health, 24.07.2019 13:30

Biology, 24.07.2019 13:30



Biology, 24.07.2019 13:30

Biology, 24.07.2019 13:30



Health, 24.07.2019 13:30