
Chemistry, 20.09.2019 06:20 jonmorton159
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) → p4o6 (s) ∆h = -1640 kj i. heat is absorbed ii. heat is released iii. rxn is exothermic iv. rxn is endothermic v. products have higher enthalpy content than reactants vi. reactants have higher enthalpy content than products

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) →...
Questions

Mathematics, 09.03.2021 05:50


Mathematics, 09.03.2021 05:50



Chemistry, 09.03.2021 05:50

Geography, 09.03.2021 05:50


Mathematics, 09.03.2021 05:50

Mathematics, 09.03.2021 05:50

Chemistry, 09.03.2021 05:50



Mathematics, 09.03.2021 05:50

Social Studies, 09.03.2021 05:50

Mathematics, 09.03.2021 05:50

Arts, 09.03.2021 05:50

Biology, 09.03.2021 05:50


English, 09.03.2021 05:50

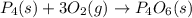
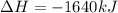
 for the reaction comes out to be negative.
for the reaction comes out to be negative.

