
Chemistry, 09.04.2020 19:25 sparky1234
A student puts 0.020 mol of methyl methanoate into an empty and rigid 1.0 L vessel at 450 K. The pressure is measured to be 0.74 atm. When the experiment is repeated using 0.020 mol of ethanoic acid instead of methyl methanoate, the measured pressure is lower than 0.74 atm. The lower pressure for ethanoic acid is due to the following reversible reaction. CH3COOH(g)+CH3COOH(g) ⇋ (CH3COOH)2(g)+Assume that when equilibrium has been reached, 50 percent of the ethanoic acid molecules have reacted. i. Calculate the total pressure in the vessel at equilibrium at 450 K. ii. Calculate the value of the equilibrium constant, Kp, for the reaction at 450 K

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2

Chemistry, 23.06.2019 08:00
How does the digestive system interact with the circulatory system? a. messages sent as electrical impulses from the digestive system are transported throughout the body by the circulatory system. b. nutrients taken in and broken down by the digestive system are carried to various parts of the body by the circulatory system. c. nutrients and gases are absorbed by organs in the circulatory system. then, they are transported to all parts of the body by organs in the digestive system. d. oxygen and carbon dioxide are exchanged by organs in the digestive system, and the gases are carried to the rest of the body by the circulatory system.
Answers: 2
You know the right answer?
A student puts 0.020 mol of methyl methanoate into an empty and rigid 1.0 L vessel at 450 K. The pre...
Questions

Mathematics, 01.12.2019 09:31

Chemistry, 01.12.2019 09:31


Mathematics, 01.12.2019 09:31



Advanced Placement (AP), 01.12.2019 09:31

History, 01.12.2019 09:31

Mathematics, 01.12.2019 09:31





Mathematics, 01.12.2019 09:31

Mathematics, 01.12.2019 09:31


English, 01.12.2019 09:31

English, 01.12.2019 09:31


Mathematics, 01.12.2019 09:31

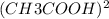 gas formed are calculated as
gas formed are calculated as


