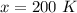Chemistry, 09.04.2020 06:28 Lizzy527663
The temperature of a gas is 100 K and its volume is 500.0 ml. If the volume increases to 1,000.0 ml,
what is the new temperature of the gas?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
The temperature of a gas is 100 K and its volume is 500.0 ml. If the volume increases to 1,000.0 ml,...
Questions



Mathematics, 09.12.2021 02:10

Mathematics, 09.12.2021 02:10

Mathematics, 09.12.2021 02:10

Physics, 09.12.2021 02:10





Mathematics, 09.12.2021 02:10



Mathematics, 09.12.2021 02:10

Mathematics, 09.12.2021 02:10

Mathematics, 09.12.2021 02:10


Mathematics, 09.12.2021 02:10


Mathematics, 09.12.2021 02:10

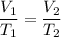
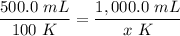 Cross-multiply:
Cross-multiply: 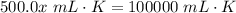 Isolate x:
Isolate x: