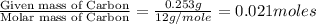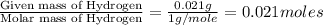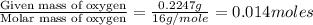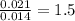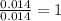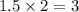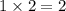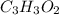Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Determine the empirical formula of the following compound:
a 0.4987-g sample of a compo...
a 0.4987-g sample of a compo...
Questions

Mathematics, 11.06.2021 03:00




Social Studies, 11.06.2021 03:00


Mathematics, 11.06.2021 03:00






Mathematics, 11.06.2021 03:00



Mathematics, 11.06.2021 03:00



Biology, 11.06.2021 03:10

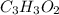
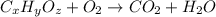
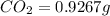
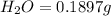
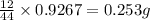 of carbon will be contained.
of carbon will be contained.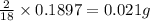 of hydrogen will be contained.
of hydrogen will be contained.