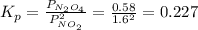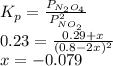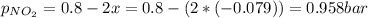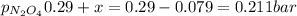N2O4 decomposes into NO2. At a certain temperature, the equilibrium pressures of NO2 and N2O4 are 1.6 bar and 0.58 bar, respectively. If the volume of the container is doubled at constant temperature, what would be the partial pressures of the gases when equilibrium is re-established

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 13:30
1. what is boyle’s law? • state the definition of the law in words. • what are the assumptions of boyle’s law? • write at least one mathematical equation that represents the law. • what can be calculated with boyle’s law? • using a gas-filled balloon as an example, describe what is happening to the gas molecules inside the balloon before and after you squeeze it.
Answers: 2
You know the right answer?
N2O4 decomposes into NO2. At a certain temperature, the equilibrium pressures of NO2 and N2O4 are 1....
Questions



Mathematics, 18.01.2021 14:00




Computers and Technology, 18.01.2021 14:00








Computers and Technology, 18.01.2021 14:00