
Chemistry, 09.04.2020 03:57 paolacorazza
15.0 mL of 0.050 M Ba(NO3)2 M and 100.0 mL of 0.10 M KIO3 are added together in a 250 mL erlenmeyer flask. In this problem, ignore ionic strength effects and neglect the possible presence of any complex ions in solution. a. Will Ba(IO3)2 precipitate out of solution? Support your answer with appropriate calculations.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1



Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
15.0 mL of 0.050 M Ba(NO3)2 M and 100.0 mL of 0.10 M KIO3 are added together in a 250 mL erlenmeyer...
Questions

Mathematics, 09.02.2020 19:21

Mathematics, 09.02.2020 19:21

Arts, 09.02.2020 19:21

Mathematics, 09.02.2020 19:22

Mathematics, 09.02.2020 19:22


Mathematics, 09.02.2020 19:22

Mathematics, 09.02.2020 19:22


Mathematics, 09.02.2020 19:24

Chemistry, 09.02.2020 19:25


English, 09.02.2020 19:34

English, 09.02.2020 19:34

Mathematics, 09.02.2020 19:35



Mathematics, 09.02.2020 19:36

Computers and Technology, 09.02.2020 19:36

Mathematics, 09.02.2020 19:36

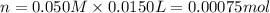
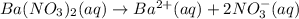

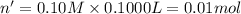
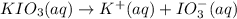
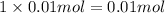
![[Ba^{2+}]=\frac{0.00075 mol}{0.250 L}=0.003 M](/tpl/images/0591/3275/f97fe.png)
![[IO_3^{-}]=\frac{0.01 mol}{0.250 L}=0.04 M](/tpl/images/0591/3275/4012f.png)
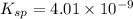
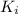
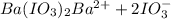
![K_i=[Ba^{2+}][IO_2^{-}]^2](/tpl/images/0591/3275/27ce4.png)
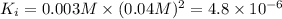
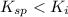 ( precipitation)
( precipitation)

