Chemistry, 09.04.2020 01:50 makemybacon
SiO32- + 2SO32-+ H2O2SO42- + Si+ 2OH-In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent. name of the element oxidized: name of the element reduced: formula of the oxidizing agent:formula of the reducing agent:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
SiO32- + 2SO32-+ H2O2SO42- + Si+ 2OH-In the above redox reaction, use oxidation numbers to identify...
Questions

Mathematics, 06.11.2019 07:31


Mathematics, 06.11.2019 07:31





English, 06.11.2019 07:31

Mathematics, 06.11.2019 07:31

English, 06.11.2019 07:31

History, 06.11.2019 07:31







Mathematics, 06.11.2019 07:31

Mathematics, 06.11.2019 07:31

Biology, 06.11.2019 07:31

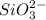 which oxidizes other but reduce itself.
which oxidizes other but reduce itself. 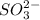 which reduces other but oxidize itself.
which reduces other but oxidize itself.