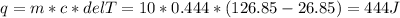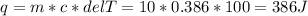Chemistry, 09.04.2020 01:32 Ashleyvasquez2261
Two 10g blocks, one of copper and one of iron, were heated from 300 K to 400K (a temperature difference of 100 K).
How much energy, in Joules, was absorbed by the iron block? *
How much energy, in Joules, was absorbed by the copper block? *

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Two 10g blocks, one of copper and one of iron, were heated from 300 K to 400K (a temperature differe...
Questions

History, 04.04.2020 01:27

Mathematics, 04.04.2020 01:27





Biology, 04.04.2020 01:27

Mathematics, 04.04.2020 01:27

History, 04.04.2020 01:27

Chemistry, 04.04.2020 01:27

Biology, 04.04.2020 01:27

Mathematics, 04.04.2020 01:27





Mathematics, 04.04.2020 01:27


Mathematics, 04.04.2020 01:27

Social Studies, 04.04.2020 01:27