
Chemistry, 08.04.2020 22:29 ayoismeisalex
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number of moles of SrCl2 is consumed when 54 g of ZnCl2 is produced?
a) 0.16 b) 0.3 c) 0.79 d) 1.58 e) 0.4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1

Chemistry, 23.06.2019 14:30
When does phenolphthalein turn pink? in the presence of a base in the presence of an acid when it is in a neutral solution when it is reacting with a metal
Answers: 1
You know the right answer?
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
Questions

Health, 18.12.2020 16:50

Biology, 18.12.2020 16:50




Mathematics, 18.12.2020 17:00

Chemistry, 18.12.2020 17:00

Mathematics, 18.12.2020 17:00




Computers and Technology, 18.12.2020 17:00








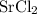 . consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.
. consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.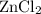 consumes 1 mole of
consumes 1 mole of 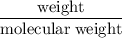
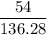 mol
mol


