
Chemistry, 08.04.2020 19:25 angelaisthebest1700
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total pressure of the gases above an equilibrium mixture if, at equilibrium, PHI = 0.010 × PH2 S?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total p...
Questions



Mathematics, 01.10.2019 07:30

History, 01.10.2019 07:30

Biology, 01.10.2019 07:30


Mathematics, 01.10.2019 07:30

History, 01.10.2019 07:30

Mathematics, 01.10.2019 07:30



Geography, 01.10.2019 07:30


Geography, 01.10.2019 07:30

Biology, 01.10.2019 07:30

History, 01.10.2019 07:30


English, 01.10.2019 07:30


Mathematics, 01.10.2019 07:30

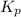
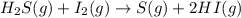
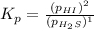
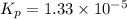
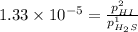
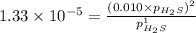
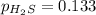
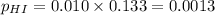
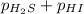 = 0.133+0.0013 = 0.1343 atm
= 0.133+0.0013 = 0.1343 atm

