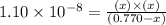Chemistry, 08.04.2020 19:24 jasmine2919
For the reaction below, Kc = 1.10 × 10⁻⁸. What is the equilibrium concentration of OH⁻ if the reaction begins with 0.770 M HONH₂?
HONH₂ (aq) + H₂O (l) ⇌ HONH₃⁺ (aq) + OH⁻ (aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
For the reaction below, Kc = 1.10 × 10⁻⁸. What is the equilibrium concentration of OH⁻ if the reacti...
Questions

Mathematics, 21.10.2020 14:01



Biology, 21.10.2020 14:01

Physics, 21.10.2020 14:01


Advanced Placement (AP), 21.10.2020 14:01


English, 21.10.2020 14:01

Physics, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01


Mathematics, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01


Biology, 21.10.2020 14:01

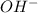 at equilibrium is 0.000092 M
at equilibrium is 0.000092 M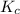
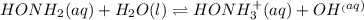
![K_c=\frac{[HONH_3^+]\times [OH^-]}{[HONH_2]}](/tpl/images/0589/8195/4bf94.png)