Given the reaction represented by the balanced equation:
CH4(g)+3F2(g)->3HF(g)+CHF3 c...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1


Chemistry, 23.06.2019 05:30
Scientist think that animals with remarkably similar embryo development probably shared a common ancestor
Answers: 1
You know the right answer?
Questions

Biology, 08.12.2020 01:30



Physics, 08.12.2020 01:30


Mathematics, 08.12.2020 01:30




Mathematics, 08.12.2020 01:30

Mathematics, 08.12.2020 01:30

History, 08.12.2020 01:30

Law, 08.12.2020 01:30





English, 08.12.2020 01:30


Mathematics, 08.12.2020 01:30

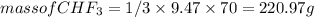
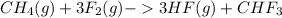
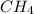 ) is taken excess amount
) is taken excess amount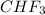 depend only on mass of fluorine
depend only on mass of fluorine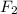 =180 g
=180 g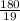 =9.47
=9.47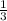 mole of
mole of 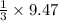 mole of
mole of 

