
Chemistry, 08.04.2020 19:04 vanydparis
Vinegar is a chemical used in cooking, cleaning and other common experiences. A 0.1 M solution of vinegar in water has a [H+] of about 1.3 × 10–3. (You may prefer to think of the hydronium ion concentration, [H3O+], as 1.3 × 10–3.)
A. Write the formula for the calculation of pH, and then show each step as you calculate the pH of a 0.1 M solution of vinegar.
B. Is vinegar an acid or a base? Explain how you know.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3


Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 09:00
Individuals within populations exhibit some diversity. as a result of possessing slightly different traits, some individuals are better able to survive and reproduce than others. if these individuals changes in the characteristics of the population may occur over time. the cumulative change in these characteristics is known as
Answers: 3
You know the right answer?
Vinegar is a chemical used in cooking, cleaning and other common experiences. A 0.1 M solution of vi...
Questions

History, 14.01.2020 10:31

Computers and Technology, 14.01.2020 10:31

Mathematics, 14.01.2020 10:31


Chemistry, 14.01.2020 10:31


Computers and Technology, 14.01.2020 10:31

Social Studies, 14.01.2020 10:31




English, 14.01.2020 10:31

History, 14.01.2020 10:31

Spanish, 14.01.2020 10:31

Chemistry, 14.01.2020 10:31





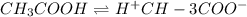
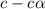
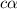
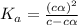
![[H^+]=c\times \alpha](/tpl/images/0589/7489/4fc41.png)
![[H^+]=0.1\times \alpha](/tpl/images/0589/7489/b5870.png)
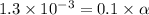
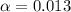
![pH=-log[H^+]](/tpl/images/0589/7489/15713.png)
![pH=-log[1.3\times 10^{-3}]=2.9](/tpl/images/0589/7489/92802.png)


