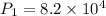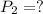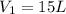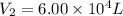Chemistry, 08.04.2020 17:17 clydeben4543
A 15.0 L tank of gas is contained at a high pressure of 8.20*10^4. The tank is opened and the gas expands into an empty chamber with a volume of 6.00*10^4 L. Calculate the new pressure of the gas.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1
You know the right answer?
A 15.0 L tank of gas is contained at a high pressure of 8.20*10^4. The tank is opened and the gas ex...
Questions


Mathematics, 29.07.2019 18:00


Mathematics, 29.07.2019 18:00

History, 29.07.2019 18:00

Biology, 29.07.2019 18:00





Biology, 29.07.2019 18:00

Mathematics, 29.07.2019 18:00

Mathematics, 29.07.2019 18:00


Mathematics, 29.07.2019 18:00

Health, 29.07.2019 18:00

Mathematics, 29.07.2019 18:00


English, 29.07.2019 18:00

English, 29.07.2019 18:00

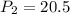
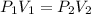 ..........1
..........1