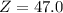Chemistry, 08.04.2020 05:03 munozjosue258
Chromium has four naturally occurring isotopes. Chromium-50 has a percent abundance of 4.35%, chromium-52 has a percent abundance of 83.79%, chromium-53 has a percent abundance of 9.50%, and chromium-54 has a percent abundance of 2.37%. Based on this information calculate the average atomic mass of chromium. *

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Chromium has four naturally occurring isotopes. Chromium-50 has a percent abundance of 4.35%, chromi...
Questions


Mathematics, 08.01.2022 18:20

Mathematics, 08.01.2022 18:20

Mathematics, 08.01.2022 18:20

Mathematics, 08.01.2022 18:20

History, 08.01.2022 18:20


Mathematics, 08.01.2022 18:20



Mathematics, 08.01.2022 18:20

English, 08.01.2022 18:20




Mathematics, 08.01.2022 18:30


Mathematics, 08.01.2022 18:30


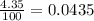
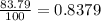
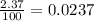
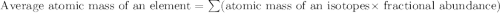
![Z=[(50\times 0.0435)+(52\times 0.8379)+(54\times 0.0237)]](/tpl/images/0589/0146/846f5.png)