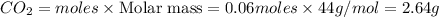G Gaseous methane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 0.96 g of methane is mixed with 6.37 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
G Gaseous methane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water...
Questions


Computers and Technology, 05.10.2019 03:00

Mathematics, 05.10.2019 03:00

Computers and Technology, 05.10.2019 03:00


Geography, 05.10.2019 03:00

Biology, 05.10.2019 03:00







Mathematics, 05.10.2019 03:00



Mathematics, 05.10.2019 03:00

History, 05.10.2019 03:00

History, 05.10.2019 03:00

Mathematics, 05.10.2019 03:00

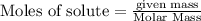
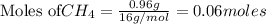
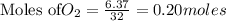
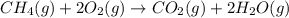
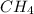 require = 2 moles of
require = 2 moles of 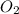
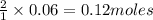 of
of 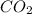
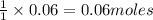 of
of