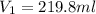Chemistry, 08.04.2020 04:33 damonsmith201615
A chemist must prepare of aqueous calcium sulfate working solution. He'll do this by pouring out some aqueous calcium sulfate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in of the calcium sulfate stock solution that the chemist should pour out. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 10:20
Determine the mass of the object below with accuracy and to the correct degree of precision. a. 324.2 g b. 324 g c. 324.30 g d. 324.25 g
Answers: 3
You know the right answer?
A chemist must prepare of aqueous calcium sulfate working solution. He'll do this by pouring out som...
Questions


English, 28.07.2019 06:30


Geography, 28.07.2019 06:30

Geography, 28.07.2019 06:30

Geography, 28.07.2019 06:30

Geography, 28.07.2019 06:30



Social Studies, 28.07.2019 06:30

Social Studies, 28.07.2019 06:30





Chemistry, 28.07.2019 06:30




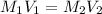
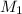 = molarity of stock calcium sulphate solution = 1.82 M
= molarity of stock calcium sulphate solution = 1.82 M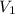 = volume of stock calcium sulphate solution = ?
= volume of stock calcium sulphate solution = ?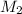 = molarity of dilute calcium sulphate solution = 1.00 M
= molarity of dilute calcium sulphate solution = 1.00 M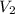 = volume of dilute calcium sulphate solution = 400 ml
= volume of dilute calcium sulphate solution = 400 ml