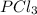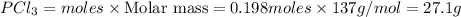For the following reaction, 6.12 grams of phosphorus (P4) are mixed with excess chlorine gas . The reaction yields 21.5 grams of phosphorus trichloride . phosphorus (P4) ( s ) chlorine ( g ) phosphorus trichloride ( l ) What is the theoretical yield of phosphorus trichloride

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

You know the right answer?
For the following reaction, 6.12 grams of phosphorus (P4) are mixed with excess chlorine gas . The r...
Questions






Geography, 07.03.2020 00:40


History, 07.03.2020 00:40

History, 07.03.2020 00:40




Chemistry, 07.03.2020 00:40







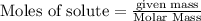
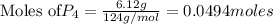
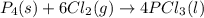
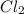 is the excess reagent,
is the excess reagent, 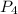 is the limiting reagent as it limits the formation of product.
is the limiting reagent as it limits the formation of product.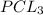
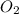 give =
give =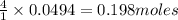 of
of