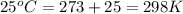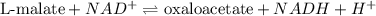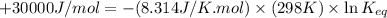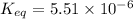Chemistry, 08.04.2020 03:26 pinkmoonlight
In the citric acid cycle, malate is dehydrogenated to oxaloacetate in a highly endergonic reaction with a ΔG’o of +30 kJ mol-1: L‐malate + NAD+ ⇌ oxaloacetate + NADH + H+ Calculate the equilibrium constant K’eq of this reaction. What is the implication of this result?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

You know the right answer?
In the citric acid cycle, malate is dehydrogenated to oxaloacetate in a highly endergonic reaction w...
Questions

History, 21.04.2020 02:34




Mathematics, 21.04.2020 02:34





Mathematics, 21.04.2020 02:35



History, 21.04.2020 02:35


History, 21.04.2020 02:35

Computers and Technology, 21.04.2020 02:35




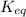 of this reaction is,
of this reaction is, 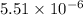
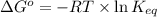
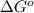 = standard Gibbs free energy = +30 kJ/mol = +30000 J/mol
= standard Gibbs free energy = +30 kJ/mol = +30000 J/mol