
Chemistry, 08.04.2020 03:30 joselaboyNC16
What is the final acetate ion concentration when 69. g Ba(C2H3O2)2 is dissolved in enough water to make 970. mL of solution? The molar mass of Ba(C2H3O2)2 is 255.415 g/mol.
0.28 M
0.64 M
0.91 M
0.20 M
0.56 M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
What is the final acetate ion concentration when 69. g Ba(C2H3O2)2 is dissolved in enough water to m...
Questions

Mathematics, 28.06.2019 15:30



History, 28.06.2019 15:30

Chemistry, 28.06.2019 15:30

English, 28.06.2019 15:30

Geography, 28.06.2019 15:30


Computers and Technology, 28.06.2019 15:30

History, 28.06.2019 15:30

English, 28.06.2019 15:30




Arts, 28.06.2019 15:30

Arts, 28.06.2019 15:30

History, 28.06.2019 15:30

History, 28.06.2019 15:30

Mathematics, 28.06.2019 15:30

History, 28.06.2019 15:30

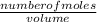
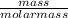
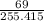 = 0.27moles
= 0.27moles 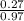 = 0.28M
= 0.28M

