

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Stock solutions of nitric acid have concentration of 16.0 M. What volume of stock solution is needed...
Questions

Mathematics, 08.01.2020 06:31



Chemistry, 08.01.2020 06:31

Chemistry, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31

English, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31


Mathematics, 08.01.2020 06:31



Mathematics, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31

Advanced Placement (AP), 08.01.2020 06:31

Mathematics, 08.01.2020 06:31

Biology, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31


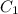 = 16 M
= 16 M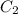 = 2 M
= 2 M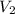 = 2 L = 2000 mL
= 2 L = 2000 mL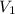
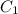 x
x 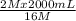 ⇒
⇒ 

