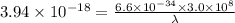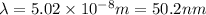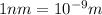Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
The first ionization energy, E , of a helium atom is 3.94 aJ. What is the wavelength of light, in na...
Questions

English, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57



Mathematics, 08.06.2020 22:57


Mathematics, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57


Mathematics, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57

Advanced Placement (AP), 08.06.2020 22:57


English, 08.06.2020 22:57



Mathematics, 08.06.2020 22:57

History, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57

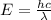
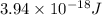
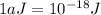
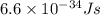
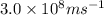
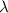 = wavelength of light = ?
= wavelength of light = ?