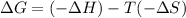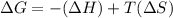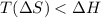Chemistry, 08.04.2020 02:29 omojay1257
For the reaction CH4(g) + 2O2(g)CO2(g) + 2H2O(g) H° = -802 kJ and S° = -5.20 J/K At standard conditions, this reaction would be product favored at all temperatures. at relatively high temperatures. at relatively low temperatures. at no temperature.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
For the reaction CH4(g) + 2O2(g)CO2(g) + 2H2O(g) H° = -802 kJ and S° = -5.20 J/K At standard conditi...
Questions


Mathematics, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30

History, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30




Mathematics, 30.11.2020 20:30





Physics, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30


Mathematics, 30.11.2020 20:30



Mathematics, 30.11.2020 20:30

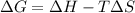
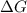 = Gibb's free energy change
= Gibb's free energy change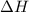 = enthalpy change
= enthalpy change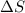 = entropy change
= entropy change