
C6H12O6 (s) + NH3 (g) -> C3H8O3 (l) + C2H6O(l) +CH1.83O0.56N0.17 (s) + CO2(g) + H2O Note that 1 mole glucose produces 0.5 mol glycerol and that the ratio of NH3 to H20 is 1:1. During preparation , you accidentally add too much glucose. However, you decide to let the bioreactor run anyway. After the reaction goes to completion at least one of the starting reactants is used up, you discover that 1.4 lb-m ethanol had been produced. How much heat had been produced and subsequently removed from the system. How much glucose did you initially add to the reactor?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
The earth's moon is unusually large. two popular theories of the moon's origin include the "sister world" hypothesis, which states that the moon formed from the same materials as the earth, near enough to the earth that they fell into orbit around each other. a second theory is the "capture" hypothesis, in which the moon formed elsewhere in the solar system, and the earth's gravity pulled it into its orbit. studies of what the moon is made of indicate that some of its materials had to come from the earth or from the same area of the solar system where the earth had formed. at the same time, the moon does not contain much of the material that makes up the earth's core, so the moon could not have formed from the same materials as the earth. how do the two facts above affect the described theories of the moon's origin? a. they show that scientists will never agree on where the moon came from. b. they show that more experiments on moon formation need to be done. c. they show that no theory accounts for the existence of the moon. d. they show that neither theory is complete and entirely correct.
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
C6H12O6 (s) + NH3 (g) -> C3H8O3 (l) + C2H6O(l) +CH1.83O0.56N0.17 (s) + CO2(g) + H2O Note that 1 m...
Questions


Mathematics, 11.02.2021 03:10


Mathematics, 11.02.2021 03:10



Mathematics, 11.02.2021 03:10



History, 11.02.2021 03:10

Mathematics, 11.02.2021 03:10

Mathematics, 11.02.2021 03:10



Mathematics, 11.02.2021 03:10





Mathematics, 11.02.2021 03:10

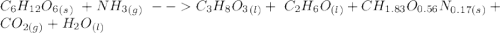
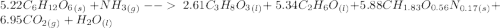
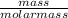
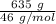
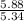 moles of S. cerevisiae formed.
moles of S. cerevisiae formed.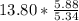 moles of S. cerevisiae formed
moles of S. cerevisiae formed

