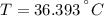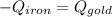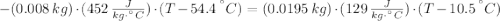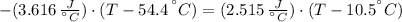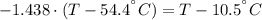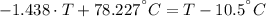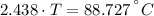Chemistry, 08.04.2020 02:07 tleppek6245
A sheet of gold weighing 8.8 g and at a temperature of 10.5°C is placed flat on a sheet of iron weighing 19.5 g and at a temperature of 54.4°C. What is the final temperature of the combined metals? Assume that no heat is lost to the surroundings.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2
You know the right answer?
A sheet of gold weighing 8.8 g and at a temperature of 10.5°C is placed flat on a sheet of iron weig...
Questions




Computers and Technology, 07.10.2021 05:30


Mathematics, 07.10.2021 05:30


Mathematics, 07.10.2021 05:30

Biology, 07.10.2021 05:30



Mathematics, 07.10.2021 05:30



Mathematics, 07.10.2021 05:30





Biology, 07.10.2021 05:30