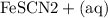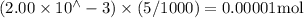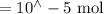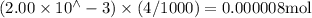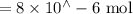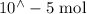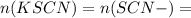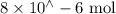Suppose a student mixes 4.00 mL of 2.00 # 10-3 M Fe(NO3 ) 3 with 5.00 mL of 2.00 # 10-3 M KSCN and 1.00 mL of water. The student then determines the [FeNCS2+] at equilibrium to be 8.75 # 10-5 M. Find the equilibrium constant for the following reaction. Show all your calculations for each step. Fe3+ (aq) + SCN- (aq) FeNCS2+ (aq) Step 1. Calculate the initial number of moles of Fe3+ and SCN- (use Equation 12). moles of Fe3+ moles of SCN- Step 2. How many moles of FeNCS2+ are present at equilibrium? What is the volume of the equilibrium mixture? mL moles of FeNCS2+ How many moles of Fe3+ and SCN- are consumed to produce the FeNCS2+? moles of Fe3+ moles of SCN-

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
Suppose a student mixes 4.00 mL of 2.00 # 10-3 M Fe(NO3 ) 3 with 5.00 mL of 2.00 # 10-3 M KSCN and 1...
Questions



Mathematics, 02.08.2019 13:00


Mathematics, 02.08.2019 13:00

Chemistry, 02.08.2019 13:00

Mathematics, 02.08.2019 13:00

Biology, 02.08.2019 13:00


Health, 02.08.2019 13:00







Physics, 02.08.2019 13:00

Chemistry, 02.08.2019 13:00


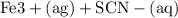 <---->
<---->