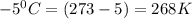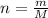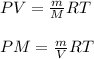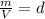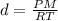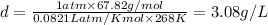Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3

You know the right answer?
Calculate to three significant digits the density of boron trifluoride gas at exactly and exactly ....
Questions


Mathematics, 01.12.2019 03:31




Mathematics, 01.12.2019 03:31

Mathematics, 01.12.2019 03:31

English, 01.12.2019 03:31

Mathematics, 01.12.2019 03:31

Health, 01.12.2019 03:31

Health, 01.12.2019 03:31







Physics, 01.12.2019 03:31