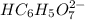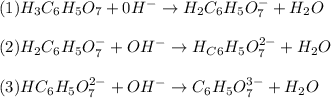Chemistry, 08.04.2020 01:44 jayline2003
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6H5O7 2- ions. What is the net ionic equation for the reaction that occurs when NaOH is added to a buffer containing H2C6H5O7 - and HC6H5O7 2- ?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6...
Questions

Mathematics, 01.12.2021 01:00

Mathematics, 01.12.2021 01:00


History, 01.12.2021 01:00


Social Studies, 01.12.2021 01:00





Business, 01.12.2021 01:00


Chemistry, 01.12.2021 01:00

Mathematics, 01.12.2021 01:00

Physics, 01.12.2021 01:00


Health, 01.12.2021 01:00

Computers and Technology, 01.12.2021 01:00


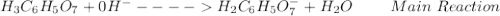
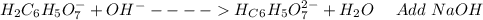
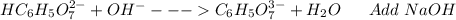
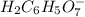 and
and