Chemistry, 08.04.2020 01:03 kenziepickup
A sample of ideal gas is heated in a 2L vessel at a temperature of 320 Kelvin. the pressure in the vessel is 2.5 atm. What is the new pressure in the vessel if the volume is halved and the temperature is reduced to 250 Kelvin?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
A sample of ideal gas is heated in a 2L vessel at a temperature of 320 Kelvin. the pressure in the v...
Questions



Chemistry, 09.06.2020 04:57

Chemistry, 09.06.2020 04:57


Mathematics, 09.06.2020 04:57


Chemistry, 09.06.2020 04:57

History, 09.06.2020 04:57





History, 09.06.2020 04:57

Mathematics, 09.06.2020 04:57



Mathematics, 09.06.2020 04:57

Mathematics, 09.06.2020 04:57

English, 09.06.2020 04:57

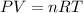
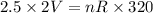 ..............(1)
..............(1)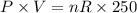 ................(2)
................(2)