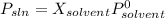Chemistry, 07.04.2020 23:47 mariamsakayanthebest
At a certain temperature, the vapor pressure of pure benzene is atm. A solution was prepared by dissolving g of a nondissociating, nonvolatile solute in g of benzene at that temperature. The vapor pressure of the solution was found to be atm. Assuming that the solution behaves ideally, determine the molar mass of the solute.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
At a certain temperature, the vapor pressure of pure benzene is atm. A solution was prepared by di...
Questions




Mathematics, 25.02.2020 00:05







Geography, 25.02.2020 00:05