
Chemistry, 07.04.2020 23:53 audrey1256
1. Sulfuric acid is formed when sulfur dioxide gas reacts with oxygen gas and water. Write a balanced chemical equation for the reaction. If 12.5 mol sulfur dioxide reacts, how many mol of sulfuric acid can be produced and how many moles of oxygen gas is needed

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
1. Sulfuric acid is formed when sulfur dioxide gas reacts with oxygen gas and water. Write a balance...
Questions

SAT, 24.11.2020 19:20

English, 24.11.2020 19:20


Health, 24.11.2020 19:20



Arts, 24.11.2020 19:20


Business, 24.11.2020 19:20



SAT, 24.11.2020 19:20


Mathematics, 24.11.2020 19:20

Mathematics, 24.11.2020 19:20

Mathematics, 24.11.2020 19:20


Mathematics, 24.11.2020 19:20


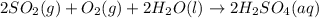
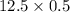 mole of oxygen
mole of oxygen

